valency of strontium|Strontium valence electrons : Tagatay Subscribed. 2. 628 views 1 year ago Electron Configuration. Valence electrons are those electrons present in the outermost shell of an atom. In this video, we will see two ways by which one can. Japanese Porn Videos! - Asian, Japanese Uncensored, Hentai Porn - SpankBang
valency of strontium,Valence : 1: Hydrogen (-1), +1: 2: Helium: 0: 3: Lithium +1: 4: Beryllium +2: 5: Boron-3, +3: 6: Carbon (+2), +4: 7: Nitrogen-3, -2, -1, (+1), +2, +3, +4, +5: 8: Oxygen-2: 9: Fluorine-1, (+1) 10: Neon: 0: 11: Sodium +1: 12: Magnesium +2: 13: Aluminum +3: 14: . 56. 12K views 3 years ago. There are two ways to find the number of valence electrons in Strontium (Sr). The first is to use the Periodic Table to figure out . The electron configuration for the valence electrons in strontium is [Kr] 5s 2. The valence electrons are the electrons in the outermost energy level, which in this case .
Subscribed. 2. 628 views 1 year ago Electron Configuration. Valence electrons are those electrons present in the outermost shell of an atom. In this video, we will see two ways by which one can.The valency of an element is a measure of its combining capacity and can be defined as. the number of electrons that must be lost or gained by an atom to obtain a stable electron configuration. What does the term .Element Strontium (Sr), Group 2, Atomic Number 38, s-block, Mass 87.62. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. As we all know Strontium is a chemical element in the chemistry branch of science. It has the representative symbol of Sr and the atomic number 38. Flerovium . Strontium has 2 valence electrons. Methods. We can write the valence electrons of strontium using two different methods: #1 Using periodic table. #2 Using . Strontium has 2 valence electrons because there are 2 electrons present in the outermost shell of the Strontium (Sr) atom. Now let’s see how you can easily find the .

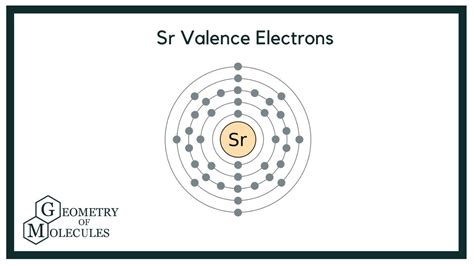
Strontium is a chemical element of the periodic table with chemical symbol Sr and atomic number 38 with an atomic weight of 87.621 u and is classed as a alkaline earth metal. . Valence electrons : 2: Valency electrons : 2: Bohr model: Electron shell for Strontium, created by Injosoft AB Sr. Figure: Shell diagram of Strontium (Sr) atom. So, as the strontium element is present in group 2, it has 2 valence electrons. In this way, by knowing the position of strontium element in periodic table, you can easily find its valence electrons. Now let’s see another method for finding the number of valence electrons in strontium. Method 2: From the Electron Configuration
The ground state electron configuration of strontium is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 5s 2. This electron configuration shows that the last shell of strontium has two electrons. Therefore, the valence electrons .The atomic number of strontium is 38, which means it has 38 electrons. Now it is possible to find the orbital notation of strontium very easily through electron configuration. That is, the orbital notation of strontium is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2.Strontium's atomic number is 38. It's a member of the alkaline earth metals, which are the elements in family IIA on the periodic table. Any element in families IA - VIIIA are main group elements. Valence electrons: For main group elements (i.e s-block and p-block elements), the valence electrons are the electrons present in the outermost orbit. But for most of the transition and inner transition elements, the valence electrons are the electrons present in the shells outside the noble gas core.
There are 38 electrons in Strontium. These elements are present in 5 orbits of the shell. The distribution of electrons is 2, 8, 18, 8, and 2. There are 2 electrons located in the outermost shell of the Strontium so the number is valence electron is 2. Strontium Number of Valence Electrons. A number of valence electrons in Strontium is 2.valency of strontiumThe outermost shell of an atom is called the valence shell, and the electrons in that shell are called the valence electrons. Strontium (Sr) has 38 electrons. K-shell has 2 electrons, L-shell has 8 electrons, M- shell has 18 electrons, N shell has 8 electrons, and valence shell has 2 electrons. The electron configuration of strontium is [ Kr] 5s 2. In the above electron configuration, the highest energy level (5) is marked with green color. The 5 th energy level contains 5s subshell and it has 2 electrons. So strontium has a total of 2 valence electrons. Next: Cesium valence electrons.

Strontium is a naturally occurring element found in rocks, soil, dust, coal, and oil. Naturally occurring strontium is not radioactive and is either referred to as stable strontium or strontium. Strontium in the environment exists in four stable isotopes,84Sr (read as strontium eighty-four),86Sr,87Sr,88Sr. Strontium compounds are used in making .Strontium is a naturally occurring element found in rocks, soil, dust, coal, and oil. Naturally occurring strontium is not radioactive and is either referred to as stable strontium or strontium. Strontium in the environment exists in four stable isotopes,84Sr (read as strontium eighty-four),86Sr,87Sr,88Sr. Strontium compounds are used in making .
Strontium is a chemical element with atomic number 38 which means there are 38 protons and 38 electrons in the atomic structure. The chemical symbol for Strontium is Sr. Electron Configuration and Oxidation States of Strontium. Electron configuration of Strontium is [Kr] 5s2. Possible oxidation states are +2. Electron Configuration
Strontium is the element of group 2 in the periodic table and its symbol is ' Sr '. The atomic number of strontium (Sr) is 38. The electronic configuration of strontium is [2, 8, 18, 8, 2]. Since it has 2 electrons in its valence shell, hence its valency is 2. Hence, the symbol of Strontium is Sr and its valency is 2.valency of strontium Strontium valence electrons In this case, the strontium atom carries a positive charge. Sr – 2e – → Sr 2+. Here, the electron configuration of strontium ion (Sr 2+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6. This strontium ion (Sr 2+) has thirty-eight . As we all know Strontium is a chemical element in the chemistry branch of science. It has the representative symbol of Sr and the atomic number 38. Flerovium Valence Electrons. Plutonium Valence Electrons. Mercury Valence electrons. Lead Valence electrons. Tellurium Valence Electrons. Gold Valence Electrons. Nobelium .Strontium valence electrons The oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive number) to form a chemical bond. A lithium atom has one outer shell electron. It has a valence of 1. Usually it’s oxidation state is +1, but it can lose the electron and have a valence of -1. The most stable oxidation state is one that .
5 Strontium and zinc are both metals with a valency of 2. Strontium is more reactive than zinc. Its chemistry is similar to that of calcium. (a) (i) Complete the following table that shows the number of protons, electrons and neutrons in each particle. particle protons electrons neutrons 88 Sr 90 Sr 65 Zn 2+ [3] (ii) Explain why 88 Sr and 90
Mg2+Cl−Cl− (4.3.2) (4.3.2) Mg 2 + Cl − Cl −. Now the positive and negative charges are balanced. We could write the chemical formula for this ionic compound as MgClCl MgClCl, but the convention is to use a numerical subscript when there is more than one ion of a given type— MgCl2 MgCl 2.Valence (valency) ( ) Lithium Li Hydrogen H 1 1 Sodium Na Fluorine F 1 Potassium K Chlorine Cl Rubidium Rb 1 Bromine Br 1 3 5 7 1 Cesium Cs Iodine I Francium Fr Oxygen O 2 ( 1) Silver Ag Sulfur S Amonium NH4+ Selenium Se 2 4 6 2 Beryllium Be Tellurium Te Magnesium Mg Nitrogen N 1 3 5 3 Calcium Ca (2 4) Strontium Sr 2 Phosphorus P
valency of strontium|Strontium valence electrons
PH0 · Valency Chart (Valency Table of Chemical Elements)
PH1 · Valences of the Elements Chemistry Table
PH2 · Strontium valence electrons
PH3 · Strontium Valence Electrons (And How to Find them?)
PH4 · Strontium Valence Electrons (And How to Find them?)
PH5 · Strontium Valence Electrons
PH6 · Strontium (Sr) Valence Electrons and Electron
PH7 · Strontium
PH8 · How to Find the Valence Electrons for Strontium (Sr)?
PH9 · How to Find the Valence Electrons for Strontium (Sr)
PH10 · Complete Electron Configuration for Strontium (Sr, Sr2+)